How to Prepare for Your 2022 CMS CPE Audit – Or Your CMS Program Audit!!
It’s never too early to prepare your organization for a Centers for Medicare and Medicaid Services (CMS) Compliance Program Effectiveness (CPE) Audit. Fear not! Segal Medicare Experts (SME) is here to help; especially if you have not had a CMS Program Audit in the last three years!!! Is your entire organization ready? Because it IS a team effort, and not just one for the compliance department!
Can your organization achieve the “perfect” score for the CMS CPE Audit?
CMS Program Audits can occur at any time during the year. Regardless of what kind of audit for which you may be selected, the key is “Always Be Prepared”!!
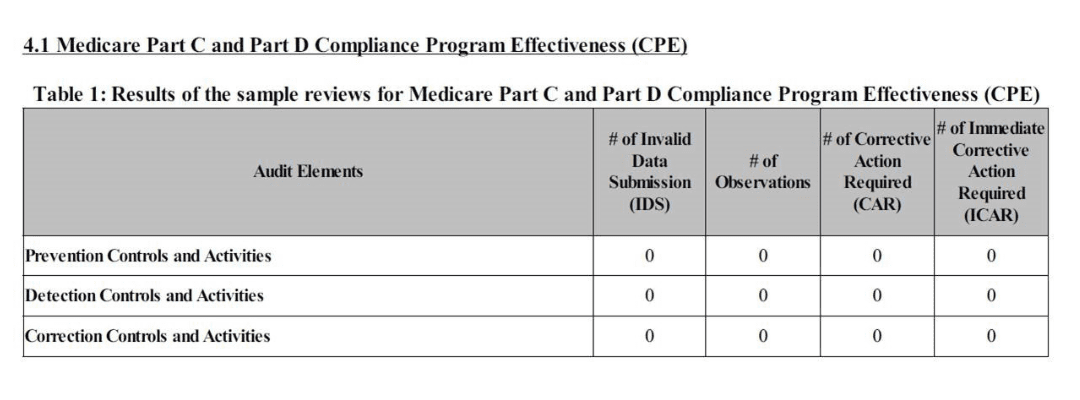
Within the CPE Program Audit, CMS will be assessing 3 areas:
✓ Prevention controls and activities – Processes, metrics, and oversight activities in place to identify a trend towards noncompliance as well as intervention taken to correct and/or mitigate.
✓ Detection controls and activities – Processes, metrics, and oversight activities to internally identify noncompliance in a timely manner.
✓ Correction controls and activities – Processes to quickly respond to noncompliance, including root cause analysis, impact assessment, communication, and corrective action.
There are several ways CMS assesses these controls and activities, as well as elements of a Sponsoring Organization’s (SO’s) compliance program. Through a combination of documents, questionnaires, overview presentations, work plans, organizational charts, risk assessments, data universes, reporting, and interviews, CMS will gain a comprehensive understanding of the effectiveness of your compliance program.
Prepare by engaging Segal Medicare Experts to assist in focused mock audits and reviews. SME helps numerous SOs prepare for CPE audits and knows how to communicate processes clearly and comprehensively.
Segal Medicare Experts
- Includes the types of documentation CMS expects;
- Shows how to effectively compile narratives; and,
- Provides interview preparation tools and sessions.
Here are the CMS Lessons Learned for Sponsors
“To help sponsors strengthen their overall compliance programs, and to benefit the program more broadly, we are summarizing some of the observations we made during our analysis of 2021 enforcement referrals.
- Medicare Parts C and D Plan-Directed Care
Sponsors are reminded that they can only hold their enrollees financially liable for the applicable cost-sharing when a contracted provider refers an enrollee to a non-contracted provider for a service that is covered by the plan (also known as “plan-directed care”). Claims processors should be properly trained and adhere to the established procedures for identifying plan-directed care before out-of-network claims are denied. This may include providing claims processing staff with increased training and/or job aids. There should also be sufficient oversight of denied claims, in particular, to ensure non-contract providers are given the appropriate appeal rights and waiver of liability statement in their denial notices.
- Monitoring for Enrollee Overcharges
We recommend that sponsors improve their internal processes for monitoring and refunding (when appropriate) overcharges to enrollees by contracted and non-contracted providers. Improved monitoring and analysis of claims denials, co-pays/co-insurance coding, and provider payments (both contracted and non-contracted) could improve a sponsor’s ability to identify overcharges that require correction. Sponsors must ensure that enrollees are not overcharged and, when they are, refunds are issued to enrollees for any incorrectly collected amounts. We may impose a CMP on sponsors when enrollees have been overcharged or there was a substantial likelihood that enrollees were overcharged.”
Here Are The Five Phases Of A CMS Program Audit As Stated By (CMS) The Center For Medicare Services In Their 2022 Report
Phase I: Audit Engagement and Universe Submission
The Audit Engagement and Universe Submission phase is the six-week period prior to the field work portion of the audit. During this phase, a Sponsoring organization is notified that it has been selected for a program audit and is required to submit the requested data, which is outlined in the respective Program
Audit Protocol and Data Request document. Key milestones within Phase I include:
Engagement Letter – The Auditor-in-Charge (AIC) conducts a courtesy call to the Sponsoring
organization’s Medicare Compliance Officer to notify the organization of the program audit. After the phone call, the AIC sends an audit engagement letter via the Health Plan Management System (HPMS).
The engagement letter contains instructions for downloading important audit documents from the HPMS.
Attached with the engagement letter is the Audit Submission Checklist2, which identifies all universe requests and deliverables due to CMS prior to the start of audit field work. The scope of each universe request is different for each program area. The CPE universe requests a list of all compliance oversight activities that occurred during the 26-week period preceding and including the date of the audit engagement letter. FA and CDAG universes are based on a Sponsoring organization’s MAPD/PDP enrollment. ODAG universes are based on a Sponsoring organization’s MA/MAPD enrollment. The SNP and MMPCC universes request all enrollees as of the date of the audit engagement letter. Finally, the SARAG universes are the 12-week period prior to and including the date of the engagement letter. CMS reserves the right to expand the review period to ensure sufficient universe size.
Engagement Letter Follow-Up Call – Within 2 business days of the date of the engagement letter, CMS conducts a follow-up call with the Sponsoring organization. The purpose of this call is to provide an opportunity for the Sponsoring organization to ask questions about the engagement letter and audit process, as well as for CMS to emphasize important information within the engagement letter and outline next steps in the audit process.
Program Area Follow-Up Calls – Within 5 business days of the date of the engagement letter, CMS conducts universe follow-up calls for each audited program area. The purpose of these calls is to answer any questions the Sponsoring organization may have regarding the data request and supplemental documentation files requested in the respective Program Audit Data Request documents.
Pre-Audit Issue Summary – Within 5 business days of the date of the engagement letter, the Sponsoring organization is asked to provide a list of all disclosed issues of noncompliance that are relevant to the program areas being audited and may be detected during the audit. A disclosed issue is one that the Sponsoring organization reported to CMS prior to the date of the audit engagement letter. Issues identified by CMS through on-going monitoring or other account management/oversight activities during the plan year are not considered disclosed. Sponsoring organizations should provide a description of each disclosed issue as well as the status of correction and remediation using the Pre-Audit Issue Summary (PAIS) template found in the HPMS. The Sponsoring organization’s Account Manager will review the PAIS to validate that disclosed issues were known to CMS prior to the date of the audit engagement letter.
Universe Submission – Within 15 business days of the date of the engagement letter, the Sponsoring organization must submit all requested universes to CMS following the instructions in the engagement letter, Audit Submission Checklist, and each respective program area Audit Process and Data Request document.
A blank version of the Audit Submission Checklist is posted on the CMS Program Audit Website located at:
Segal Medicare Experts
SME@SMEteam.org
OR 562.334.7980
segalmedicareexperts.com